Research - Laboratory for Surface Science
The main research topics include:
-
oxide formation on metal or alloy surfaces by low-energy ion implantation
The ion-induced oxidation represents a feasible alternative to thermal oxidation of metals, as it eliminates the need for elevated temperatures. In our studies, we use a low-energy oxygen bombardment (with the O2+ ions in 0.5-5 keV energy range), for the oxidation of various metals (chromium, cobalt, nickel, iron, titanium, molybdenum) or alloys (nitinol, cobalt-chromium- molybdenum). X-ray photoemission spectroscopy (XPS) is an ideal technique for determining the chemical state of a given element on the sample surface and calculating the relative concentration of certain oxidation state. We compare our experimental results with the various theoretical models of metal oxidation, to determine the kinetics of the oxide growth on the metallic surface.
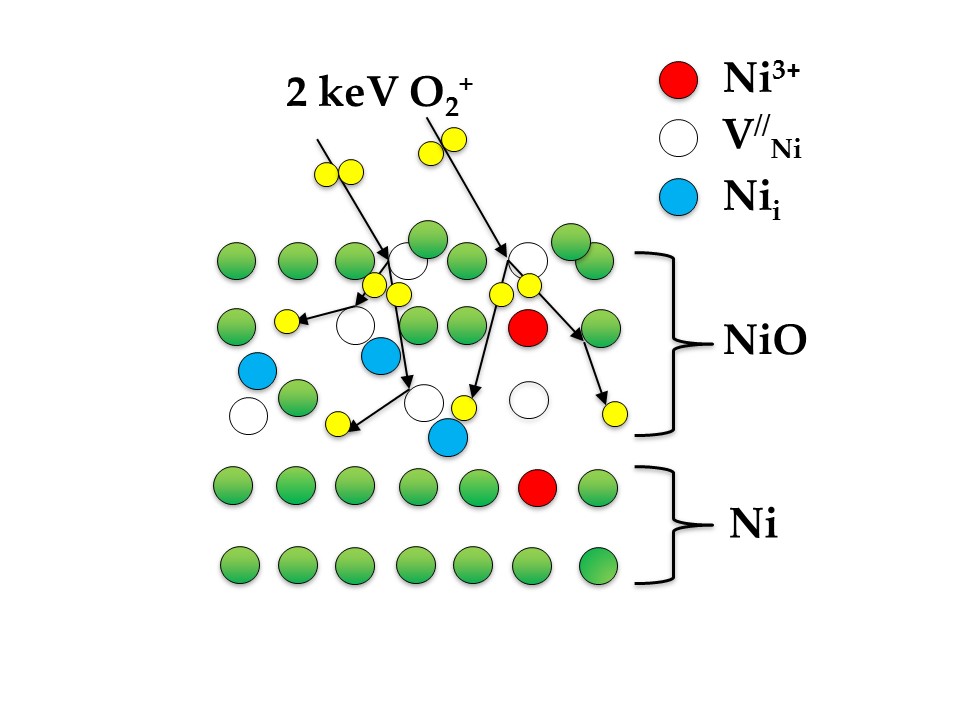
Shematics of thin oxide film growth on Ni surface by O2+ ion bombardment
-
characterisation of the impurities and point defects in semiconductor or insulating films
During the synthesis of semiconductor or insulating films by different deposition techniques (for instance atomic layer deposition or magnetron sputtering deposition), there are always some impurities left in the matrix of the materials (i.e. hydrogen in zinc oxide or chlorine in titanium dioxide). Concentration and type of impurities or point defects can have a key role in properties of materials, such as conductivity or the crystal structure. Secondary ion mass spectrometry (SIMS) can detect very small concentrations of impurities in the material and determine its depth profile (distribution) in the sample. On the other hand, XPS can be employed to determine the chemical bonding of a given element in the material.
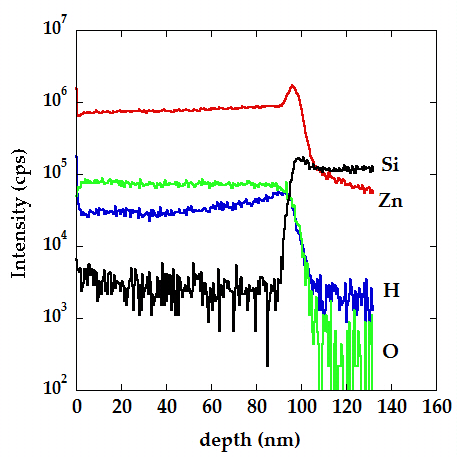
SIMS depth profile of ZnO thin film grown on Si substrate, showing the distribution of
hydrogen impurities through the film
-
examination of the metallic surfaces modified by electrochemical methods
Electrochemical techniques present an efficient method in modification of a metallic surfaces or a growth of a thin passive film on the material. For instance, growth of a tin sulphate on a tin surface or a nickel oxide/hydroxide is possible when introducing these metals to certain electrolytic solutions. Using our experimental techniques, chemical composition, elemental analysis and thickness of electrochemically formed thin films on metallic surfaces are successfully determined.
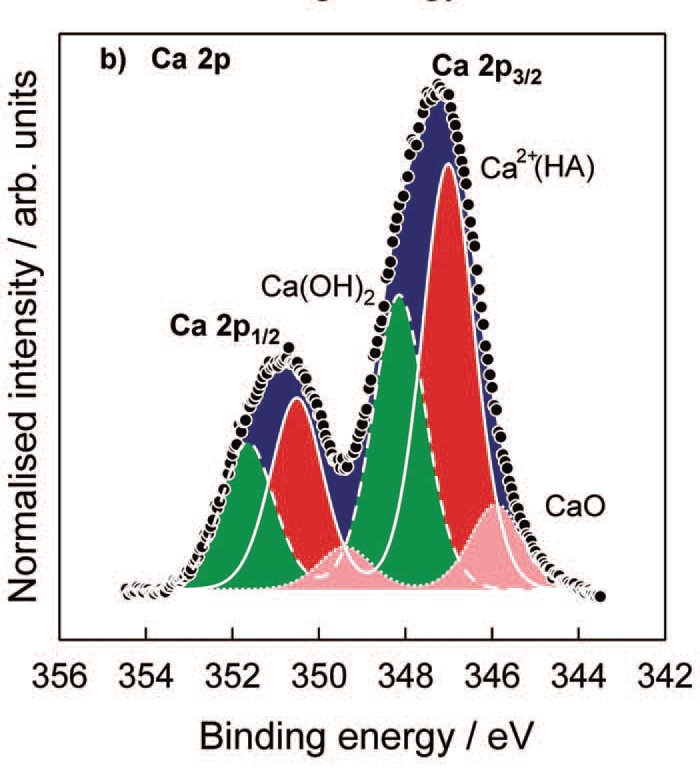
XPS spectrum around Ca 2p atomic level meassured on the hydroxiapatite (HA) film
grown on the Mg-Al-Zn alloy